User interviews《RORZE Lifescience, Inc.》Acquiring huge amounts of data and optimizing cell culture conditions.
“The productivity of skin sheets for treating vitiligo has almost tripled, and the contamination rate has decreased by five digits.”

RORZE Lifescience, Inc.
Main Business field
Production of life science related products
Interviewee
President & CEOYukito Yamasaki
Adopted equipment/systems
*This product has been discontinued. If you have any questions about cell cultures, assays, or products, please contact us using the contact form.

RORZE Lifescience Inc. (hereinafter RORZE LS) continuously contributes to automation in fields such as drug discovery research, iPS cell research, and regenerative medicine through its automation technology and experience in the semiconductor industry.
Nikon's BioStudio-mini has been adopted in RORZE LS's "Cell Keeper series" cell culture system which has an automatic medium exchange function and its "Cell Shot series" automatic observation system, through highly accurate visualization of the cell culture process. This contributes to standardization of the cell culture procedure and regenerative medicine using skin sheets.
We interviewed Mr. Yukito Yamasaki, the company’s President and CEO, about the discoveries and innovations realized by using BioStudio-mini.
Contents
ShowRORZE LS innovation

Paving the way for standardization by automating cell culture.
RORZE LS is developing and commercializing automatic equipment in the fields of drug discovery screening and regenerative medicine based on mechatronics technology that is also applied to semiconductor manufacturing equipment. What made you enter the life sciences market?
Originally, I personally specialized in mechanical design and equipment development, and was involved in the development of semiconductor manufacturing equipment. The opportunity to get involved in the life science market was the ‘protein crystallization robot system’ that was commissioned in 2002. After that, I created a screening system for researching drug discovery. Based on those technologies, in March 2011, we participated in the development of the automation system Tissue Factory (T-Factory*)for the manufacture of cell processing products, and were mainly in charge of the transportation mechanism. Since that time, and based on this experience, we have been developing automatic cell culture equipment in the field of regenerative medicine.* One of the Japanese Cabinet Office's cutting-edge research and development support programs: “the Funding Program for World-Leading Innovative R&D on Science and Technology” (FIRST). Tissue Factory for the Creation Factory (T-Factory) provides system integration for industrializing regenerative medicine.
Were there any challenges stepping into such unknown territory?
Indeed, there were. I had started by creating culture systems without knowing much about cells, and our equipment generally did not function effectively in the beginning. However, we persevered without cease until everything operated properly, and that attitude has led us to the present moment, I believe. T-Factory was also the beginning of our relationship with Nikon. I was very grateful that people around us, including those at Nikon, understood that we were not sure of ourselves and patiently instructed us about various aspects of what we were trying to achieve. In the other direction, they also adopted our ideas which were quite different from their conventional ones, so we were able to progress with development at a reasonable cost.
Contributing to vitiligo treatment with a skin sheet culture system.
What did you learn from developing T-Factory?
You have adopted the BioStudio Series for both systems.
Yes. I believe the BioStudio Series started with T-Factory. Nikon’s engineering staff created the first BioStudio model for us in order to fulfill our requirements, which were that it should be able to be incorporated into equipment and decontaminated. We had worked on the development of automatic observation systems for inspecting wafer defects and detecting contaminating materials within semiconductor manufacturing equipment, but Nikon’s optical system was superb.
Thank you. Recently, you launched a joint company with Shanghai ReMed Biotechnology Co.Ltd . (hereinafter ReMed) in China, and together have been developing a cell culture system that manufactures cell sheets for use in vitiligo treatment. I’ve heard that clinical application is progressing.
Challenges prior to introduction
Tiled image acquired with the BioStudio-mini in CellKeeper 48 Plus.
The challenge is to control contamination and variations.
Having many successful treatments is a great strength. I imagine you encountered various challenges in achieving stable cell sheet manufacturing for regenerative medicine. What aspects were particularly difficult?
There were two things in particular. One was how to minimize contamination. The other was how to achieve stable manufacturing, controlling the variation in cultures using a patient’s own cells (autologous cells).
How did you solve these problems?
Regarding the contamination reduction, the greatest source of contaminants is people, so the most effective measure is to remove them from the process. However, complete automation is not available. Therefore, we control ventilation to prevent contaminants (particles) from reaching cells. Another concern is that no machine or equipment operates completely without issue. Considering the fact that accidents do occasionally occur, such as power outages caused by lightning, for example, redundancy is built into our system design to aid recovery.
For autologous cells, may the culture schedule be changed?
Yes, that may happen. Particularly when using autologous cells, the growth rate up to the first passage varies widely. Sometimes, extremely rare changes that would not generally be considered the norm in chemical synthesis, such as altering the parameter time freely, are made, extending the culture time by one day because growth is insufficient, for example. We are taking care to employ a flexible approach, including software that can respond to these situations.
Developing an automatic cell culture system while learning about the behavior of cells.
We spoke about variation in cells, but what is necessary for the stable production of cells?
Unfortunately, this can lead to poor development. To help improve this situation, in April 2018, we established a joint research course at Osaka University and decided to continue development while learning as much as we could about the behavior of these complex systems called cells, and what is actually required to handle them. We were able to acquire a lot of data at the university and verify the optimal culture method for realizing stable cell production. Through digitizing culture methods, we aimed to move beyond the so-called ‘Meister’-like method.
The BioStudio-mini is indispensable for establishing cell culture engineering.
What is the ‘Meister’-like method you mentioned?
I see. But in terms of examination, we can’t really quantify the manual operation, such as how and to what extent the cells are agitated, can we?
For the cell culturing process, we have combined CellShot, which is equipped with the BioStudio-mini, and CellKeeper 48 Plus, which unites an automatic incubator and a media exchange system. This allowed us to create a mechanism that can flexibly combine processes while monitoring their progress. As a result, even if the characteristics of the cells used as the starting material vary, the culture can be performed with good yield. In this way, we have been able to nearly triple our productivity to produce skin sheets for vitiligo treatment and reduce the contamination rate by five orders of magnitude.
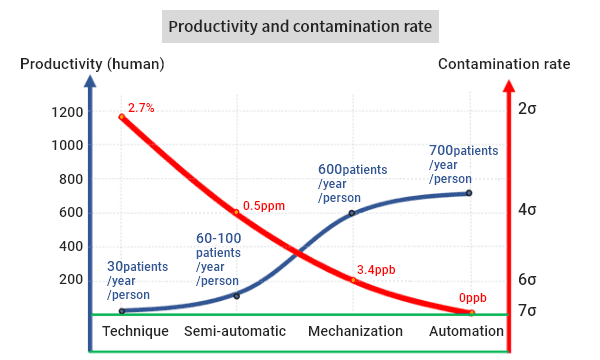
The less human intervention there is, the lower the chance of contamination will be. Semi-automation*1 realized by CellKeeper 48 Plus reduced the contamination rate by five orders of magnitude, from 2.7% to 0.5ppm. Regarding productivity, cell proliferation is a biological phenomenon. There is an upper limit to the speed, but it is greatly improved by mechanization*2 and automation*3 compared to manual work.
*1 Semi-automation: The protocol is performed by humans and machines in cooperation.*2 Mechanization: The entire protocol is performed by machines, but human judgement and manual work are applied as necessary.
*3 Automation: The entire protocol is performed without human intervention.
Effects of introduction

The BioStudio-mini integrated into the CellShot 48Plus
An excellent optical system, compactness, and flexibility were the deciding factors for adoption.
Your company has integrated the BioStudio-mini as the observation system in the CellShot and CellKeeper. What was the deciding factor?
There were actually three main reasons. The principal factor was that only the BioStudio-mini could properly observe iPS cells. We inquired with other companies about their optical system algorithms, but when we compared their algorithms and their actual way of viewing, only Nikon’s product could effectively observe iPS cells and acquire observation subject coordinates. Since we were developing and selling defect inspection systems for semiconductor wafers, we were capable of installing components such as x-y stages, and we possessed know-how about image software and tiling functions. Therefore, we were able to complete CellShot by integrating the BioStudio-mini.
The second reason was that the optical system was extremely compact.
Compact size was also a deciding factor?
That’s right. The development concept for CellKeeper was to use 60cm square units with various functions, combining like toy blocks. Therefore, the BioStudio-mini with its compact optical unit was ideal for installation in a limited space. There were not any serious issues with its systemization, but if I had to raise a concern, a challenge was the effect of temperature change on cells because you need to move them outside of the incubator when capturing images while performing full-scale tiled acquisition. However, we were able to solve this problem by adding temperature and CO2 controls as standard functions within the CellShot module, which is equipped with CellKeeper.
The third reason was the flexibility of Nikon’s engineers. Based on BioStudio, they were able to heavily modify the interface. We were able to have conversations between engineers and they proceeded to completely satisfy our requirements. Without that response, the product we have now would not have been possible. I am very grateful that we were able to develop as One Team.
Thank you. Is it related to the fact that CellShot realizes tiled acquisition while using the BioStudio-mini, which does not have a native tiled acquisition function?
That’s correct. To achieve tiling, information is exchanged between our device stage and the BioStudio-mini, and they repeatedly cooperate to perform actions, which means capturing images when culture containers move. Nikon developed this information exchange into a DLL (Dynamic Link Library). Generally, it is controlled via communication, but combining programs was faster and more stable. I imagine this kind of request might usually be rejected. However, thanks to their positive response, we were able to successfully acquire live images. Also, by integration with a controlling software we developed, we were able to control the image position with an accuracy of one micron, including axial (Z) precision. For this reason, CellShot was able to realize extremely high-precision movement during tiled acquisition.
Tiled acquisition and time-lapse imaging provide data that overturns conventional wisdom.
So how do you actually use it?
We utilize 48 culture containers (SBS standard, 100 mm dishes) that are loaded into the unit. By capturing tiled images for every plate, it has become possible to perform time-lapse analysis for the entire area within the containers. We have now achieved the macroscopic observation of cell distribution inside the culture containers and it has become possible to observe how the seeded cells are changing within the containers.
I heard that your company has recently adopted Nikon’s image analysis software. What do you think of it?
It’s an essential tool. The amount of tiled image data captured by CellShot is enormous. No one would want to manually plot the coordinates of cells in thousands of images, right? [laughs]
I certainly wouldn’t want to, and even if I did, I would probably make mistakes.
Probably so. Not only that, but because the work is being carried out by human eyes, results will vary from person to person. Even if the same worker performs the task, the result may change depending on their physical condition each day. Such data cannot be effectively used for analysis. However, if this image analysis is automated, a single index can be used as a fixed standard, delivering reliable results.
Accumulating high-quality data that can be used for creating indicators is important for the industrialization of cell culture.
Yes, I think so. Employing CellKeeper enables stable cell culture by integrating the culture process, media exchange, and observation, while also automating image analysis . As a result, we are able to clarify how various operations affect the cell culture, such as shaking the plate vertically three times after inoculation. It has brought us some surprising results.

The first step to cell culture engineering.
What kind of data did you capture? Please tell us.
With cultured MSCs (1,136 strains derived from newborns and 1,146 strains derived from the elderly), we have acquired time-lapse video of changes in cell distribution within culture vessels with and without various operations over seven days using CellKeeper. Doing this, we understood that whatever actions were taken, nothing was changing macroscopically from when the cells were first introduced. We released these findings at the ISBF (The International Society for Biofabrication) online event ‘Call for Abstracts for the 2021 Showcase.’
That’s an incredible discovery. You mean that despite cells moving a little at the beginning, this is within a limited range, and their distribution does not fundamentally change from the time when cells are introduced?
That’s correct. For example, a cell suspension is poured into the center of the dish. When this is performed, the density of the cells is high in the center but lower toward the edges. This state doesn’t change even if you turn the dish.
Is it because they move to the center when it’s turned?
They actually don’t spread out. They don’t change position. On the contrary, when the suspension is poured in from the side and turned, you might assume they would gather toward the center, but they just stay where they were poured.
Does that mean it’s important to lay cells evenly in the beginning?
That’s right.
That’s a big surprise!
Yes, it’s quite astonishing. By employing CellShot equipped with the BioStudio-mini, all the data is captured automatically. The reproducibility is extremely high because experiments are performed without human intervention. Indeed, it feels like we have taken the first step from the ‘Meister’ method to cell culture engineering.
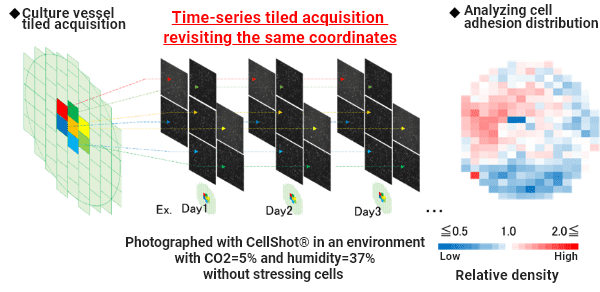
The cell distribution in the entire vessel over time is evaluated by combining tiled image acquisition and time-lapse imaging, revisiting the same coordinates.
Future expectations

Aiming for true automation of cell culture systems.
On the frontline of medical treatment, you are working on various developments with a sense of speed. Could you tell us anything about your newest projects?
We have previously established mechanical functions for 1. culture (growth), 2. media exchange, 3. observation, and 4. analysis. In the future we intend to optimize culture conditions through the unified management of these functions and by further fine-tuning the technology for each of them.
As part of our latest activities, we are engaging in development and testing with MSCs (mesenchymal stem cells) at a lab in Osaka University in order to apply a subculturing system to practical use. Following that, ReMed will conduct testing using skin cells, and we will complete the work using feedback from clinical data.
After the subculturing system has been completed, we will add “5. Subculture” to the previous four functions, and furthermore, by implementing AI, we aim to achieve true automation of our cell culture systems. We also intend to advance the technological development of pretreatment and aftertreatment.
Creating a new market for cell culture automation.
You are breaking into territory that still has no market, aren’t you?
Yes, that is the case. The automation of cell culture that we are working on still does not have a market. Therefore, we are taking on the challenge from the standpoint of creating the market and the culture for it. It’s sometimes known as the ‘searching for a blue ocean strategy’, but checking market reports is not really that useful. This is because when new markets are written about in such reports, they have already been discovered. So, in fact we need to create our own ‘blue ocean strategy’. Looking at it from this perspective, I think that what we actually possess is extremely valuable and fresh information.
Nikon definitely wants to share these discoveries and work together with you to contribute to the development of cell-based and regenerative medicines. Thank you very much for this valuable conversation.
*All information is current as of the time of the interviews.*From April 2021, sales of the BioStudio series are being conducted by Nikon Solutions Co., Ltd. Nikon Corporation takes charge of consultations such as those concerning integration into equipment.
Related cases
Nikon will contribute to solving your cell culture issues with its image analysis
techniques and know-how on cell quality evaluation.
Click hereInquiry Form