Types of Cell Observation with Microscope
When observing cultured cells, it is important to observe cells non-invasively because the cells are alive. This section introduces typical optical observation methods and explains the points to keep in mind for each of them.
Advantages of Non-Staining Method and Fluorescent Staying Method
Microscope observation of cells can be broadly divided into two types: non-staining and fluorescent staining.
Non-Staining Method
This is a method in which a colorless and transparent cell specimen is illuminated with transmitted light without being stained, and the properties of light are used to contrast the image. Non-staining methods include the phase contrast observation method, the differential interference observation method and the modulation contrast method and such. The unstained transmission observation method is generally less invasive compared to the fluorescence observation method in which a fluorescent dye is introduced into cells, and it has the advantage in that the observed cells can proceed to the next step in regenerative medicine.
Fluorescent Staining Method
This is a method in which a fluorescent dye is introduced into the site to be observed in the specimen, and the fluorescent light generated by irradiating illumination light through the objective lens is captured to form an image. Fluorescence observation is somewhat invasive because fluorescent labels are introduced but has the advantage of visualizing cell function expression by antibody staining.
Types of Microscope Observation Methods used in Cell Culture
❶Bright Field Observation
This is the most basic observation method for observing the specimen with transmitted light. Image contrast is represented by the difference in light transmittance, and color information is represented by the wavelength of transmitted light. The purpose of using bright-field observation is to (1) ascertain the approximate location and density of cells as a pre-stage for more detailed observations such as phase contrast observation and to (2) ascertain the morphology by staining specimens such as pathological specimens. This is the most basic optical configuration of a microscope.
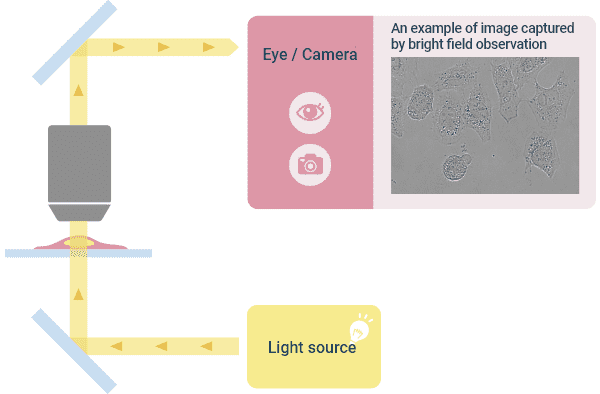
Optical Principle of “Bright Field Observation” and ImagesExample of how a typical upright microscope appears
❷Phase Contrast Observation
This observation method is suitable for observing colorless and transparent cultured cells with good contrast. Cells are transparent, but the light changes its phase (the position of its waveform) depending on where it passes through the specimen. Therefore, by devising the illumination light and combining the “direct light” whose phase does not change and the “diffractive light” whose phase does change in the image plane, contrast will be enhanced by the interference of waves and the morphology of the specimen will be displayed. As a result, even if the culture cell is colorless and transparent, an image in which the morphology is recognizable can be obtained.
The feature of phase contrast observation is that the image quality is stable because there is no directionality in the shading. Therefore, lately it is used very often for microscopic image analysis. The drawback is that you get a bright border called a halo. Nikon's proprietary “anodization phase-contrast” objective lens reduces this halo and enables clear identification even when cells are close to each other. The microscope configuration requires a condenser on the illumination side and an objective lens for phase contrast observation with a phase ring and phase film in the lens.
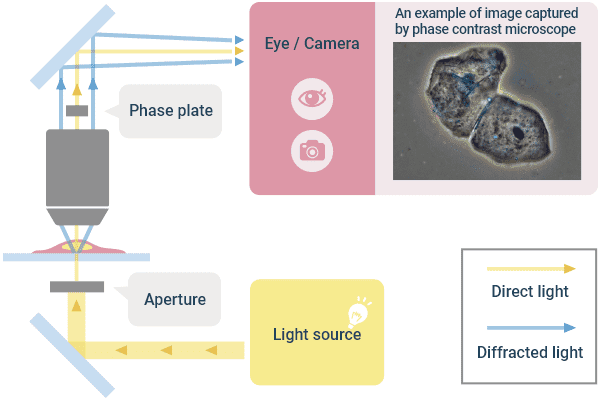
Optical Principle of “Phase Contrast Observation” and Example Image
❸Differential Interference Contrast Observation
This observation method has been devised to observe cultured cells that are phase objects with good contrast in the same manner as the phase contrast observation. The illumination light is converted to linearly polarized light using a polarizing plate, and by using a prism inserted on the illumination side, two linearly polarized light beams that are slightly separated from each other are created to illuminate the specimen. The two rays that have passed through the specimen are recombined by the prism inserted on the viewing side. Then, a phase shift occurs due to the difference in the position (thickness) of the specimen through which the two light beams have passed, and the contrast is created by interference when combined. At this time, the distance between the two rays is smaller than the optical resolution, so you do not get a double image. The image looks three-dimensional with one side of the object being dark and the other side being bright, but the directionality of shading is disadvantageous for image analysis.
Although a halo does not occur as in the phase contrast, plastic containers cannot be used for this observation method because they disturb linearly polarized light that is used. The microscope requires a polarizer and a differential interference prism on the illumination side, and a differential interference prism and an analyzer on the imaging side. Objective lenses for phase contrast cannot be used.
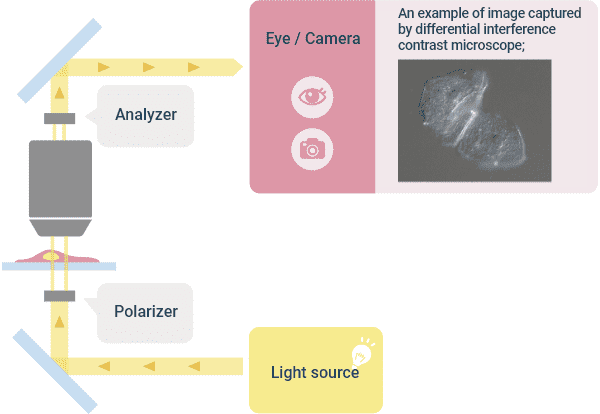
Optical Principle of “Differential Interference Observation” and Image Example
❹Modulation Contrast Observation
This is an observation method in which phase objects are visualized. A diaphragm with a slit is arranged at a position away from the center of the optical axis in the optical path of the illumination. The illumination light with an angle that passes through that slit illuminates the specimen. By guiding the light with gradient refractive index to pass through the modulation plate arranged on the imaging side, a contrast is added to the image. The image is a three-dimensional image that looks like a differential interference observation. Unlike the phase contrast observation, there is no halo and no linear polarized light is used, so plastic containers can be used with this observation method, unlike the differential interference observation. Nikon has commercialized it as the NAMC (Nikon Advanced Modulation Contrast) series using its proprietary technology.
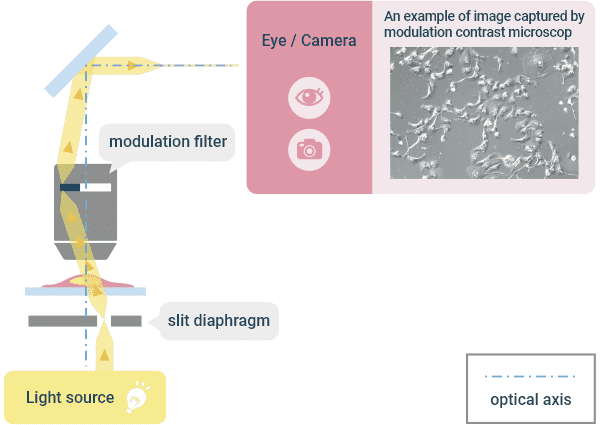
Optical Principle of “Modulation Contrast Observation” and Example Image
❺Fluorescence Observation
This method uses an immune reaction, where a specific part of a cell is stained with a dye to selectively observe that specific part only. Fluorescent molecules in fluorescent dyes exhibit a fluorescent phenomenon in which they absorb the energy of the excitation light and release the energy as light when returning to the ground state. One example that uses this fluorescence phenomenon is a “fluorescent epi-illumination method” in which excitation light is irradiated from an objective lens, and the generated fluorescence is acquired with the same objective lens. In this illumination method, the light travels back and forth in the same optical path. However, the wavelength of the generated fluorescence becomes longer than the wavelength of the excitation light (Stokes shift). This method takes advantage of this Stokes shift and separates the light into the excitation light and fluorescent light using a dichroic (color separating) filter. The transmitted light is imaged and observed visually or with a camera. Fluorescent dyes come in various wavelengths (colors), so multi-color images can be obtained by staining multiple objects with different colors. However, specimens are often fixed and then stained, so the dyes available for use for living cells are limited.
A method that has been widely used in recent years is a technique Dr. Osamu Shimomura (physicist) won the Nobel Prize in Chemistry for in 2008, that labels with a fluorescent protein such as GFP (Green Florescent Protein). By introducing a fluorescent protein gene into the gene at the site you want to observe, labeling can be done with relatively little damage to the living body, enabling visualization of the movement of living cells. Since the generated fluorescence has an intensity of only several tenths of thousands of the excitation light, images are usually acquired with a high-sensitivity camera. In addition, the objective lens needs to be a lens that does not emit autofluorescence from the structural members inside the lens to be compatible with fluorescence observation. Plastic containers emit autofluorescence, which may affect contrast, so they are not suitable for observation containers.
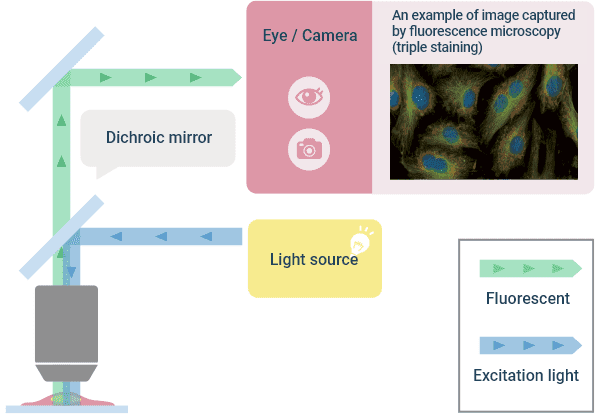
Optical Principle of “Fluorescent Observation” and Image Example

