Pluripotent Stem Cell Morphology
The undifferentiated state of pluripotent stem cells changes day-to-day, but no absolute marker for confirming pluripotency or undifferentiated state has yet been discovered. Therefore, observation of cell morphology is cited as an important approach for confirming undifferentiated state of pluripotent stem cells. Here, we discuss pluripotent stem cell morphology and how it relates to various biological characteristics.
Human Pluripotent Stem Cell (hPSC) Morphology
Inducing differentiation of pluripotent stem cells results in dramatic alterations to their morphology. Even in daily culture, where the undifferentiated state is maintained, cell morphology is different within immature colonies compared to mature colonies, and the undifferentiation markers is affected by various stimuli.
OCT-3 / 4 and NANOG expressed in undifferentiated pluripotent stem cells are used as undifferentiated markers, but they are not absolute. Therefore, it is not possible to conclude that cells expressing one of these two markers are undifferentiated. Furthermore, a marker for absolutely confirming pluripotency, that is, a pluripotent stem cell marker, has not been found. *
* There is a recent trend to rephrase “undifferentiation marker” as “pluripotent stem cell marker”, but stem cell researchers warn at workshops and other places that they should be careful not to confuse these phrases.
Therefore, several types of tests need to be performed to confirm the pluripotency of hPSCs. In addition to confirmation of the expression of undifferentiation marker genes and surface antigens, morphological characteristics are also considered to be critical indicators when identifying hPSCs 1). So, what are the characteristics of hPSCs? They are described in the Atlas of Human Pluripotent Stem cells 2) as follows:
“Undifferentiated PSC colonies typically have clear borders from the feeders and contain small round cells, without spaces between them, and large nuclei with notable nucleoli.”
Sparsely populated cell colonies start to form immediately after passage in locations where spindle-shaped or polygonal cells with well-defined outlines gather. As the days pass, cells become more densely packed, the boundaries between cells become unclear, the cytoplasm decreases significantly in volume compared to the nucleus, and prominent nucleoli become visible. As can clearly be observed by time-lapse imaging and tracking of colonies, the size and morphology of cells changes with the number of days in culture. When observing with a microscope, observe the entire colony at low magnification, then observe the cells or nuclei at high magnification. Save the images taken at both magnifications.
Morphological evaluation is very important for determining the timing of passaging. If colonies of low cell density are dissociated into single cells during passaging, cells may not adhere or differentiate after passage. Stem cell researchers usually describe low-density colonies as “young” or “immature” and high-density colonies as “mature.” When observing the culture vessel under a microscope, if there are numerous colonies with densely packed undifferentiated cells, and significantly fewer small or low-density colonies, but there are cell-free areas on the bottom of the culture vessel, then it is time to passage the culture. Microscope observation of cells is thus very important for hPSC culture.
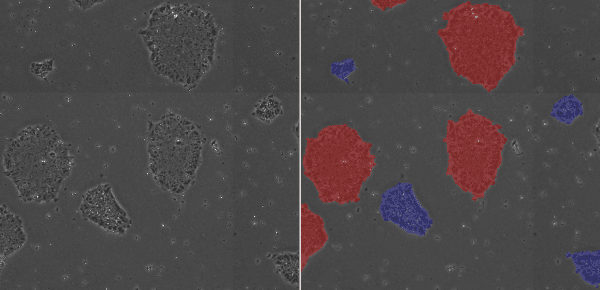
Low-Density Colony (blue), High-Density Colony (red)
The Relationship between hPSC Morphology and other Biological Characteristics
Various cell types make up the body, and there are various types of cultured cells derived from them. Each cell type has its own distinctive morphology. In general, epithelial cells have a cuboidal shape, mesenchymal cells have a large ratio between the lengths of the long and short sides of the cell, and blood cells have a round shape. However, these characteristic forms can change. Well-known examples are the epithelial-mesenchymal transition (EMT), and the other way around via the mesenchymal-epithelial transition (MET). Epithelial cells that originally had a cuboidal shape are transformed into long and spindle-shaped mesenchymal stem cells. Such transformations occur numerous times during development to form tissue 3). One of the most famous examples is the EMT occurring during neural crest (NC) formation. Neural crest cells (NCCs) transform from neuroepithelial cells into ectoderm-derived mesenchymal cells by EMT and migrate into the body cavity. The EMT is known to play a big role in cancer cell metastasis and invasion. During the EMT, expression of E-cadherin decreases and EMT-related genes such as Snail, Slug, and Twist are expressed 4~7). Expression of these genes is induced by TGF-β and Wnt signaling, or by growth factors such as EGF and FGF. Stimuli from the surrounding environment changes cell morphology. EMT has also been reported to be involved in the spontaneous differentiation of hPSCs 8~13). As the periphery of packed hPSC colonies begin to differentiate, the expression of Snail and vimentin can be observed.
Time-lapse imaging of hPSC differentiation into neural crest cells (NSCs) in 2D culture has revealed that nestin-positive cells may gather and form a colony-like state, and then expand outwards 14). From this, it is assumed that the EMT is occurring, and it was confirmed that E-cadherin expression is reduced, vimentin expression is increased, and Slug expression is also temporarily increased 15).
As described above, it is clear that cell morphology and colony shape reflect intracellular signaling.
Mesenchymal Stem Cell (MSC) Morphology
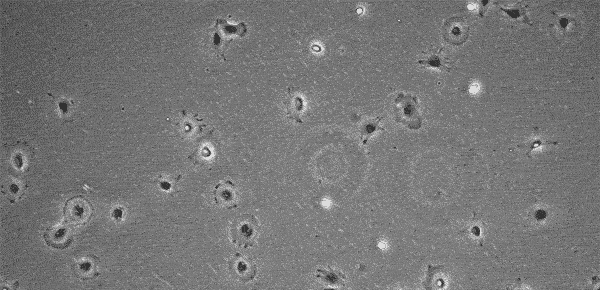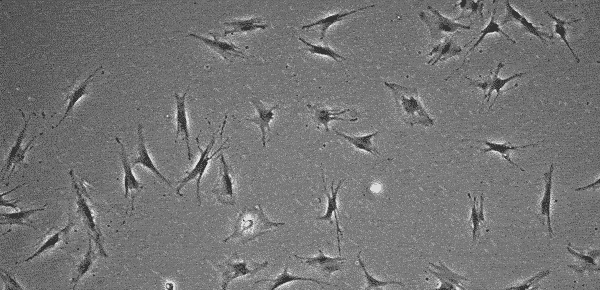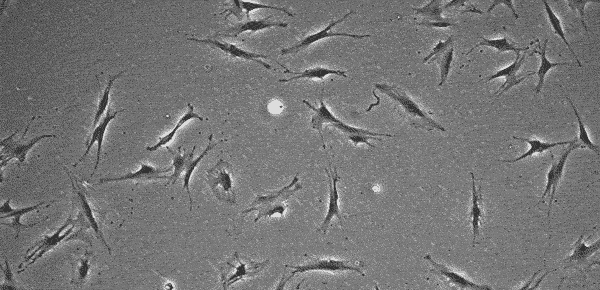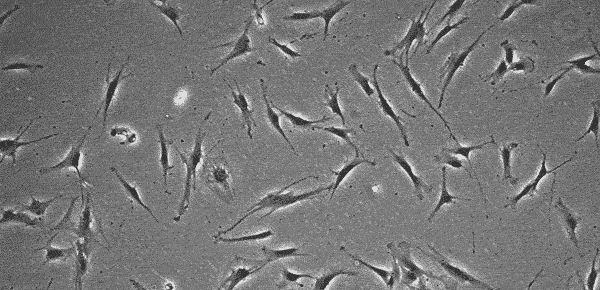
MSCs do not grow indefinitely like hPSCs, but age with each passage, with cell morphology changing in step. Several research articles have shown that at low passage numbers there are "small, spindle-shaped, fast-growing cells" and more mature "slowly growing large and flat cells" 16~18). The International Society for Cellular Therapy (ISCT) has defined that MSCs must express markers such as CD105, CD73, and CD90 19). However, since the quality of cells (whether they are proliferating or not) cannot be determined even when these markers are expressed, MSC quality evaluation methods are being discussed internationally 20). In addition, MSC characteristics vary significantly according to the original tissue and donor, and even at a low passage number cells may not proliferate, prohibiting further passage. At this point, it is up to the operator to determine at what stage the culture should be interrupted.
Storing images of the culture process and quantifying cell proliferation through image analysis makes it easier to develop clear criteria for judgment and realize strong process control. Image analysis is thus expected to be utilized in quality control.
References
1) Stem Cell Rev and Rep. 2009;5:301-314
2) Atlas of Human Pluripotent Stem Cells: Derivation and Culturing Stem Cell Biology and Regenerative Medicine, New York, Humana Press, 15-39, 2012 (ISBN 978-1-61779-548-0)
3) Cell. 2009;139:871–890
4) J Cell Sci. 2003;116:499-511
5) Cell. 2004;118:277-279
6) Mol Cell. 2001;7:1267-1278
7) J Biol Chem. 2001;276:27424-27431
8) Mol Hum Reprod. 2008;14:169-179
9) Mol Hum Reprod. 2007;13:21-32
10) Stem Cells. 2008;26:2777-2781
11) Cancer Res. 2007;67:11254-11262
12) Stem Cells and Development. 2014;Vol.23, No.18
13) PLoS One. 2013;8: e54122
14) Biol. 2018; 62: 613-621, video S1, S2
15) Int. J. Dev. Biol. 62: 613-621 (2018)
16) Mech Ageing Dev. 1981;16:81-89
17) Proc Natl Acad Sci USA. 2001;98:7841-7845
18) Biol. 2011;55:181-187
19) Cytotherapy. 2006; 8: 315–317
20) FORUM. 2014;VOLUME 14,ISSUE 2:141-145

