The Significance and Challenge of Culturing Cells
Cultured cells are now used not only for basic research, but also for industry and medicine, where expectations for stability are high, both in terms of the quality of cultured cells and the reproducibility of results. There is a growing debate about what must be done to grow good cells.
Improving Cell Culture Practices
In the EU and the US, Good Cell Culture Practice (GCCP) 1) and Best Practices in Cell Culture 2) have been proposed to ensure the reproducibility, reliability, and accuracy of cell Culture for fields such as drug discovery. These recommendations describe the basic knowledge and operations involved in culturing cells. Further, you also need the ability to observe and judge cells, as well as the culture techniques and know-how to maintain cell characteristics.
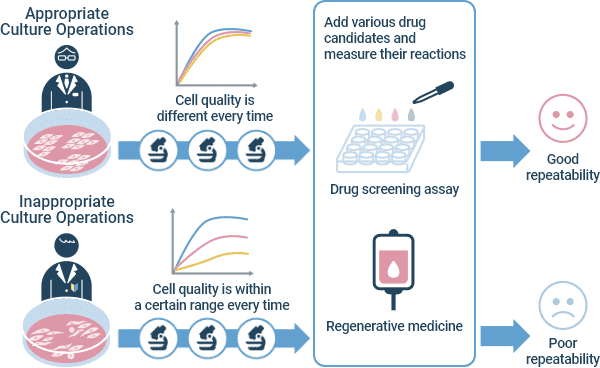
Appropriate and Inappropriate Culture Operations
Cells have various shapes depending on their origin. For example, keratinocytes which are the proliferating cells derived from the basal layer of the epidermis, are cuboid-shaped cells that grow like cobblestones. However, primary keratinocyte culture is usually contaminatied with spindle-shaped dermis-derived fibroblasts. Nerve cells have a characteristic morphology in which a number of projections extend from the cell body. By observing these adherent cells, their morphological characteristics can be confirmed and the cell type can be identified with some degree of confidence.
Cultured cells must be healthy and properly prepared in order to be successfully utilized for their intended application. For example, when examining the effects of cosmetic ingredients on keratinocytes, accurate results cannot be obtained if too many fibroblasts are present in the culture, even given a large overall cell population. Additionally, the effects are different between young and senescent keratinocytes. It is difficult to equate the results of using young keratinocytes with the results of using aged keratinocytes.. In general, experiments are performed by using cells that have undergone the same number of passages. Furthermore, in the case of human primary cells, such as skin-derived keratinocytes, the quality often varies depending on the donor or the lot. Therefore, it is necessary for judgments of quality by observing cell morphology and proliferation as well.
Now, how do you evaluate the cell quality, besides observing proliferation?
As an example, cells are fixed and immunostained with anti-keratin antibodies in order to determine their identity as keratinocytes or fibroblasts, the former of which expresses keratin. However, the same cells that were analyzed by immunostaining cannot be used in the next experiment. Non-invasive observation is preferred because cell state may be evaluated without killing the cells, and is most often performed by visual observation with a phase contrast microscope.
Now, what are the things we need to be attentive to when observing?
We propose basic methods for observation using an inverted phase contrast microscope at low and high magnifications, observation period, appropriate procedures for recording and storage.
But to be more specific, how should we conduct observations of the cell’s shape, border, nucleus, cytoplasm, and other components?
Consider cell shape as an example. Keratinocytes are cuboidal. Blood cells are spherical. Nerve cells are "jagged" due to the presence of dendrites and other processes. Young mesenchymal stem cells (MSCs) are relatively small in size and grow larger as they age.
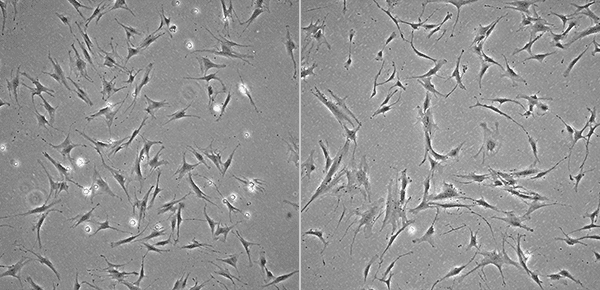
Images of Young (Left) and Senescent (Right) MSCs
Most cells have a single nucleus, but differentiated cells and senescent cells may be multinucleated. Additionally, the storage of secretions in the cytoplasm may cause swelling that pushes, flattens, and/or unevenly distributes nuclei within the cell.
Undifferentiated embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have little visible cytoplasm under phase-contrast microscopy, but in a slightly differentiated state the volume occupied by the cytoplasm increases relative to that of the nucleus. When the culture medium is not replaced at proper timing, the cell viablity decreases and their morphology changes. For example, the number and volume of vacuoles will also increase, even in HeLa cells and MSCs.
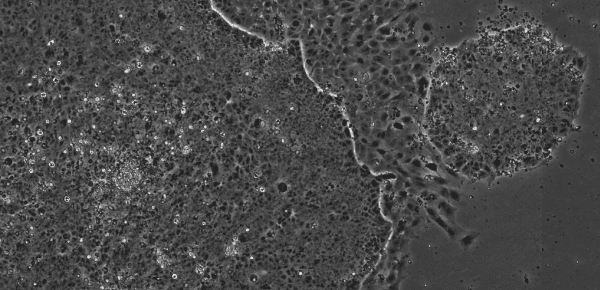
Image of iPS Cells
Unless you have a lot of experience in culturing many cell types, you likely have several questions. Fortunately, cell images are published in various places, including data sheets, cell banks, and peer-reviewed articles. Such information is useful regardless of your experience culturing a particular cell type, so it is always advisable to check.
How confident would you be as an operator with visual observation as the only tool available for assessing cell state over an extended period?
Because of the busy days in the laboratory, it’s hard to accurately remember what the cells were like yesterday, not to mention a week ago. Saving microscope images provides a permanent and quantifiable record, making it easy to compare the current cell state with the previous record and confirm if they are of acceptable quality. With this approach you can move on to the next step in your culture procedure without concern.
References
1) Cell Culture Techniques, Springer, 2011; 56: 1-25 (ISBN 978-1-61779-076-8)
2) In Vitro Cell Dev Biol Anim. 2017; 53: 669-72

