Cell Culture Assessment and Observation
Cell observation is an important component of basic cell culture procedures. With each step, the cells are observed, cell state is evaluated, and the next step in the process is determined. Determining the quality of cells generally involves fixation and immunostaining for certain marker proteins, or extraction of proteins and genetic material from cells for profiling. However, performing such tests precludes the further use of those cells, even if they are of high quality. However, it is possible to use an optical microscope to observe cultured cells in a non-invasive manner. We will take a look at the key points to consider when evaluating cell proliferation using a microscope.
Verification of Cell Proliferation
The key to successful cell culture is to focus on cell health. This is important for a number of reasons. For example, when administering a drug, healthy cells are likely to react differently than less healthy cells, affecting the results of the experiment. In order to judge cell health, one must be able to correctly determine cell state. The challenge is thus in the preparation of healthy cells, which are needed to generate reproducible results.
How is cell health judged? The simplest indicator is that cells are proliferating. Now, how does one determine if cells are proliferating without dispersing the culture and using a hemocytometer for counting?
Generally, visual observation using a phase contrast microscope is the most common method.
Related link
Phase contrast observationCells are generally transparent and colorless, making it difficult to observe morphology. Phase contrast microscopy makes use of the variable refractive index within the cellular environment, turning these differences into visually apparent contrast gradients, making it easy to observe cell morphology and certain sub-cellular components, such as the nucleus. Once you start a cell culture, you will most likely need to observe it with a microscope daily.
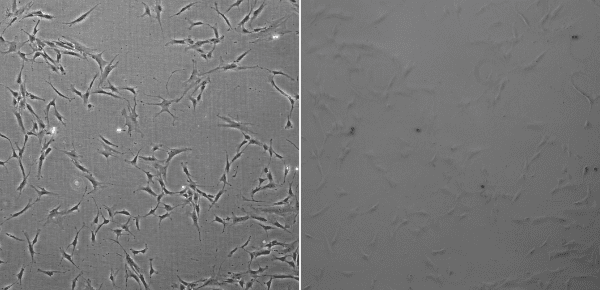
Phase contrast image with a properly aligned phase ring (left), phase contrast image without a phase ring (right)
Now, how do we determine if the cells are proliferating?
It’s simple. If there are more cells than observed previously, that means they are proliferating. First, observe the entire culture vessel. If you are culturing adherent cells, determine the relative area of the bottom surface of the culture vessel occupied by cells. You may not accurately remember the coverage area from the previous observation, so it is a good idea to acquire and store images using a microscope in order to keep a permanent record. Comparison with images from previous observations is a simple method for confirming cell proliferation.
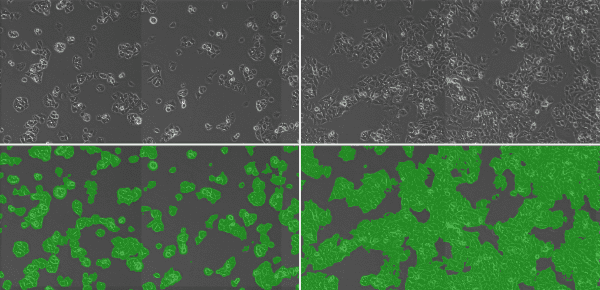
Evaluating cell proliferation over time using phase contrast imaging (top) and subsequent generation of a mask image (bottom) for calculating coverage area.
How do you assess the situation when cultured cells cease to proliferate, or have even begun to decline in number? The key is to identify and address the underlying cause. We will look at some common causes below.
① Infected with bacteria, yeast, or mold (fungi)
If cells are infected with bacteria or yeast, the color of the culture medium may be discolored, appearing yellowish and even turbid. When observed under a microscope, many small particles appear suspended in the medium. Zooming in, one might even see bacteria moving. If it is instead mold infecting the culture, its movements probably will not be visually apparent. As mold begins to grow, both the color of the medium and cell state continues to appear normal. However, by thoroughly observing the entire vessel at low magnification, one might identify radially swaying hyphae, indicating infection by mold. In the case of any type of infection, it is desirable to discard the entire culture and vessel. If it cannot be discarded, it may be possible to wash out the contaminating organisms and add antibiotics to the culture. Depending on the cells and culture conditions, however, washing and/or adding antibiotics may result in cell death, altered morphology, and other effects. One should also investigate the cause of the infection and implement appropriate process controls to address it.
② Poor Culture Environment
If the culture environment deviates from normal the cells may not proliferate, or may even die. If the culture medium takes on a more magenta hue, it may be due to a malfunction of the CO2 incubator, as decreasing CO2 concentration increases the pH of the medium, with numerous physiological consequences. Common issues include empty CO2 cylinders, low water level in the reservoir used for maintaining relative humidity, and change in temperature. If the culture environment only deviated from normal for a short time, cells might recover. However, given that cell morphology and other properties might have become permanently altered, it is desirable to discard the culture.
③ Defective Medium or Vessel
If bacteria, yeast, or mold did not infect the cells and the incubator is not malfunctioning, the only possibilities left are a defective culture medium or vessel. For the medium, it is often useful to double-check that all the appropriate supplements were added. In the case of adherent cells, it may have been caused by the culture vessel not being properly coated with an adhesion factor. In such a case, it may be possible to recover by changing the culture medium or transferring cells to a new vessel coated with the adhesion factor.
If cells are proliferating steadily without issue, cell division can be readily observed during the logarithmic growth phase, with the area occupied by cells in the vessel increasing. If the cells become confluent, occupying the entire vessel surface, then cell growth will be inhibited. In the case of cancer cells, cell growth may not be inhibited at confluency, and some cell lines may even grow to the point of becoming stratified in layers. However, there are cases where cells grow in excess and suffer from lack of nutrients, quickly resulting in the death of the entire culture. Once human normal epithelial cells grow to confluency, they may lose their proliferative potential once passaged, or may not even adhere to the vessel. Passage needs to take place before confluency is reached, which is a major reason why it is important to observe how cells are growing.

