Basic Cell Culture Processes and Procedures
Cell culture processes and procedures vary depending on the cell type and application. We need to be aware that if cells are not handled in a manner that is appropriate for each process, their characteristics might change. This section introduces general cell culture processes and procedures, while noting important points for consideration.
Preparation of Cells for Cell Culture
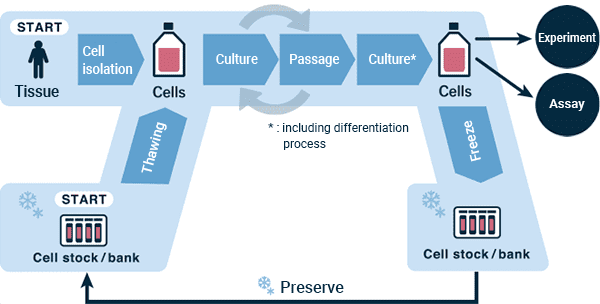
Figure: Flowchart of a generalized cell culture process
There are two methods for obtaining cells: from a cell bank or by isolating cells from donor tissue. When starting culture from cells obtained from a cell bank, one needs to go through the procedures of "thawing," "cell seeding" and "cell observation."
When using tissue collected from a donor, unnecessary tissue are usually removed if it is attached. There are two major methods to isolate cells from the tissue, explant culture and enzymatic method. In enzymatic methods, isolation of cells from the tissue of interest using a proteolytic enzyme solution. If an enzyme is used, dilute the enzyme or stop the enzyme reaction with an enzyme reaction inhibitor, then proceed with the steps of "cell seeding" and "cell observation" to prepare the cell culture.
Thawing
Thawing frozen cryopreserved cells to initiate a cell culture may be thought of as "waking up the cells." A vial of frozen cells obtained from a cell bank is transferred from a liquid nitrogen tank*1 or cryogenic deep freezer (-150 °C) to an appropriate cold storage container, such as a liquid nitrogen container, transported to the bench, and thawed in a 37°C water bath or in a melting apparatus*2 . Before ice is almost melted, medium is quickly added to dilute the cryoprotectant liquid (EX. DMSO), the cells are precipitated by centrifugation, and after removing the supernatant, fresh medium pre-heated to 37°C is added. The cells are then resuspended by pipetting and the number of cells/cell concentration is measured using a microscope or cell counter.
*1: There are two ways to freeze and preserve cells in a liquid nitrogen tank: "Vapor phase" in which liquid nitrogen's cold gas is used, and "liquid phase" in which a frozen preserved object is directly immersed in liquid nitrogen. In the case of liquid-phase, it can be preserved at -196 °C, which is the temperature of liquid nitrogen, but there is a high risk of liquid nitrogen getting into the frozen vial and contaminating it with bacteria, yeast, mycoplasma, and viruses from other vials. Therefore, storage in vapor phase is strongly recommended.*2: Vials that have been stored in the liquid phase could burst if they are placed in a 37°C -water bath or a melting apparatus with liquid nitrogen remaining in a vial. The lid should be loosened once and retightened before putting it in the 37°C -water bath.
Cell Seeding
To achieve the target cell seeding density, calculate the amount of fresh medium required to achieve the desired cell seeding density based on the measured cell numbers, and dilute the cell suspension accordingly.
Cell Observation
After seeding the cells in a new culture vessel, observe the cells in the vessel with an optical microscope or other observation device in the following manner:
- Check that there are viable cells
- Check to make sure that cells are evenly distributed in the vessel
- Check for the presence of foreign objects other than cells
- Check the cell morphology
After confirming the above, place the cell culture vessel in a humidified CO2 incubator at 37°C and start culturing.
From Cell Culture Initiation to Passage
Cell Observation
The cells are seeded in a new culture vessel, which is placed in a CO2 incubator. Generally, on the following day*, the below observations are performed using an optical microscope or other observation device:
- Check to make sure there are no foreign objects other than cells in the culture vessel
- Determine whether the culture is proceeding normally by checking cell morphology and condition*There are cases where they are left to stand for two days depending on culture conditions and cell type.
Medium Exchange
After having been thawed and seeded in a culture vessel, cells start to grow in the CO2 incubator. Cells metabolize nutrients in the culture medium, therefore medium that has been depleted of nutrients and enriched with metabolites must be replaced with fresh medium. This process is called "medium change" or "medium replacement".
Prior to medium change, first observe the cells to verify that the culture is proceeding normally. After removal of the old medium, quickly add new medium to prevent the cells from drying out. Some cells may die if not immersed in medium, so there are cases where a small amount of the old medium is left instead of being entirely discarded. Additionally, fresh medium should be pre-warmed to 37°C so as not to expose cells to sudden changes in temperature.
After changing the medium, check the cells for any signs of damage. Use a microscope to acquire images of the culture in order to document that it is proceeding well, then return it to the incubator, taking care to minimize unnecessary disturbances.
Passage
Once cells start to proliferate, divide them into new culture vessels before the current vessel becomes full. This is called "passage." The state in which cells have grown to fill the culture vessel is called "confluent." Generally, it is recommended that cells be passaged when the area occupied by cells reaches approximately 70 to 80% of the vessel.
When they become confluent, cells come into contact with each other and feel that they do not need to grow any more, a phenomenon known as "contact inhibition," and especially in the case of normal cells they will no longer proliferate after passage. In the case of cancer cells, they will continue to proliferate rapidly, but they will come to lack nutrition, so it is not good to allow them to be confluent. For this reason, it is necessary to observe and monitor cell growth.
Passage methods differ between suspension cells and adherent cells.
Passage of Suspension Cells
Collect the cell culture suspension into a tube and centrifuge to collect the cells. Remove the supernatant, leaving the cell pellet, and re-suspend in fresh medium. Take a part of the fresh suspension, apply the vital stain trypan blue, and count the number of living cells. Calculate the cell concentration, consider cell dilution methods, adjust the density of cells in suspension appropriately, and place in a new container.
Passage of Adherent Cells
Cells that adhere to the culture vessel surface need to be somehow detached from its surface. Generally, proteases such as collagenase, dispase, and trypsin are used. In the case of trypsin, activity is inhibited by calcium or magnesium ions, so it is necessary to wash out medium containing these ions prior to its application. Conversely, collagenase and dispase show activity in the presence of calcium ions.
The general procedure is as follows:
- Remove the old culture medium supernatant
- If necessary, wash with phosphate buffer or fresh medium
- Add cell dispersion enzyme solution
- Stabilize at a predetermined temperature within the active range of the enzyme for a predetermined time to promote the enzymatic reaction
- Check the degree of cell detachment under a microscope
- Promote cell detachment by mild mechanical disturbance, such as tapping, etc.
- If a stop solution for the enzyme(s) is available, add it to stop the reaction. If not, add fresh medium to dilute the enzyme and decrease activity
- Pipette the medium several times to dissociate cells into a single-cell suspension
- Collect the medium including cells, precipitate the cells by centrifugation, and remove the supernatant containing the enzyme and reaction stop solution.
- Tap the tube to loosen cell pellet
- Add fresh medium to the tube of collected cells
- Resuspend the cells by pipetting up and down
- Prepare sample as a representative cell suspension to count the cell number/cell density
- Count cell numbers using a microscope with hemocytometer or an automated cell counter
- Determine the correct dilution for obtaining the desired cell density, add the appropriate amount of fresh medium, and re-suspend the cells
- Seed a predetermined amount of the cell suspension into a new culture vessel
- Perform microscope observation
- Transfer to a humidified CO2 incubator set to a temperature of 37 °C
However, some cells could get weakened if dispersing enzymes are used. In that case, mechanically scrape and peel off cells with a scraper, or peel off cells with the flow from a pipet to harvest cells.
Processing after Cell Culture (Stock Preparation)
It is important to make stocks that have the same characteristics as the original cells, which may be obtained from primary culture, purchased from a supplier, or transferred from elsewhere. This is because cells are living materials and their characteristics may change over time and if passage continues over a long period of time, it could cause them to differ from the original cells.
A cell stock can be created as follows:
Cell Observation
Perform the following observations with an optical microscope or other observation device.
- Check for foreign objects other than cells in the culture vessel
- Determine if the cells are subconfluent and have not over-proliferated
- Determine whether the culture is proceeding normally by checking cell morphology and condition
Cell Detachment
Harvest cells for stocks using the passage procedure described earlier.
Cell Observation
Add fresh medium to resuspend the cells collected by centrifugation, but use a small amount of medium because the density of the cell suspension needs to be higher than that for passage. The cells are suspended by pipetting, and the number of cells/cell density is measured using a microscope or measuring instrument.
Dispensing
Adjust the cell suspension to the desired number of cells, add the same amount of 2x concentrated cryopreservation solution, and re-suspend by pipetting. While pipetting or stirring constantly, dispense a predetermined amount of solution into the cryotube.
Freeze
The cryotube should be immediately placed in a freezer container and placed in a deep freezer (-80° C) to keep a freezing rate of -1°C per minute. Alternatively, freeze with a programmable controlled-rate freezer. After the cells are frozen, the frozen cryotubes should be stored in the vapor phase within a liquid nitrogen storage tank or cryogenic deep freezer (-150 ° C). But, the procedures varies depending on the type of cryopreservation solution.
Confirmation
Thaw one or two of the frozen vials and culture them. Confirm whether the cells can grow in the same manner and exhibit almost identical characteristics as previously. Once this is confirmed, stop the cell culturing.
Start of Experiment
Thaw prepared cell stocks as needed for research activities. After a certain period in culture, the cells should be discarded and fresh stock thawed for use.

